Described Properties of Compressed Charcoal Briquettes
The properties described for compressed charcoal briquettes so far refer to chemical properties, but the physical properties—especially for compressed charcoal are no less important. In the iron and charcoal industries, physical properties hold significant value.
Biochar Properties for Steel Production
Charcoal for Steel Production
Charcoal is the most costly raw material in a blast furnace charge. The physical properties of charcoal affect blast furnace production, while chemical properties relate to the amount of charcoal required per ton of iron and the final composition of iron or steel.
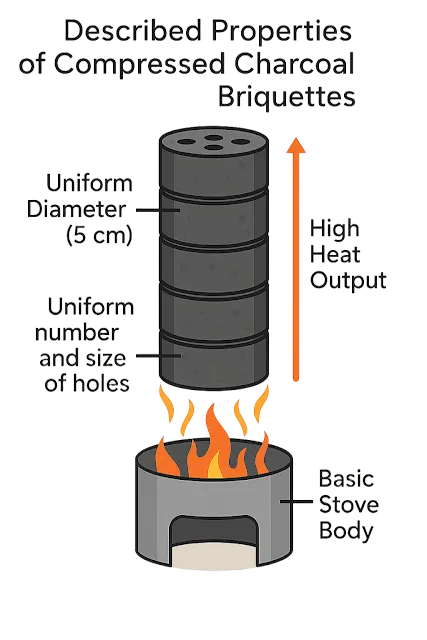
Blast furnace charcoal must be
compression-resistant to withstand the crushing load of the furnace charge.
This compressive strength always lower than that of its competitor, mineral
coal determines the practical height and, consequently, the efficiency and
productivity of the blast furnace. Resistance to breakage during handling is
critical to maintaining consistent permeability of the furnace charge to air,
which is vital for furnace productivity and process uniformity.
Physical Properties of Compressed Charcoal Briquettes
Various tests have been developed
to measure breakage resistance—a property difficult to define in objective
terms. These tests measure charcoal’s resistance to breakage or collapse by
allowing a sample to drop from a height onto a hard steel floor or by tumbling
the sample in a cylinder to determine collapse volume after a set time. Results
are expressed as a percentage passing through and retained on screens of
different sizes.
Charcoal with weak breakage
resistance will produce a higher proportion of fine particles during testing.
Fine charcoal is undesirable in blast furnaces as it impedes airflow. Brittle
charcoal can also be crushed by the charge’s weight, causing blockages.
Absorption Capacity of Charcoal
Wood charcoal is an important raw
material for activated charcoal. While this product is outside this guide’s
scope, some data may be useful, as charcoal producers sell charcoal to specialized
plants for conversion into activated charcoal.
Activated Charcoal Production
Absorption Capacity of Charcoal
Ordinary wood charcoal is not
highly absorbent for liquids or vapors because its microstructure is clogged
with tar residues. To convert charcoal into an "activated" state,
this structure must be opened by removing tar residues. The most common method
today involves heating crushed raw charcoal in a furnace to low red heat in an
atmosphere of superheated steam. The steam prevents combustion by excluding
oxygen.
Meanwhile, volatile tar is distilled off and carried away with the
steam, leaving the pore structure open. The treated charcoal is then discharged
into sealed containers and allowed to cool. Activation furnaces are typically
continuous, meaning powdered charcoal passes sequentially through the hot
furnace in a steam atmosphere.
Absorption Properties of Activated Charcoal
After activation, charcoal is
tested against quality specifications to determine its ability to decolorize
aqueous solutions (e.g., raw sugar juice, rum wine) via absorption; absorb oils
(e.g., vegetable oils); and absorb solvents (e.g., ethyl acetate) in air.
Absorption capacity tends to be specific: grades are tailored for aqueous
solutions, oils, or vapors.
Tests measure absorption capacity. Minor variations exist in the final product depending on the raw charcoal’s origin, but properly activated charcoal is generally usable. High-quality base charcoal for activated charcoal can be made from Eucalyptus grandis in brick-type kilns.
Charcoal used for gas and vapor absorption is typically made from
coconut shell charcoal. This charcoal has high absorption capacity and resists
fragmentation in absorption equipment a critical factor.

heat transfer mechanisme

Efficient Burning of Compressed Charcoal Briquettes
How to Use Compressed Charcoal Flames Efficiently
High-quality charcoal must burn
efficiently to yield optimal results, especially in household use, where most
charcoal is burned. Industrial furnaces (e.g., smelting furnaces, cupolas,
sintering furnaces) are designed and operated for efficient combustion, which
we will not discuss here. In developing countries, charcoal’s primary household
use is heating water for cooking or washing.
Some foods are cooked by direct
heating without immersion in water, such as roasting corn or meat. A cooking
system would achieve 100% efficiency if all heat released from fuel combustion
were absorbed by the food. However, this is far from reality. Well-designed and
operated equipment typically achieves ~30% efficiency, meaning 70% of heat is
wasted. In cold climates, some waste heat may be captured to warm room air,
thereby improving overall efficiency.
Heat Transfer Mechanism of Compressed Charcoal
Efficient Burning of Compressed
Charcoal Briquettes
Charcoal Heat Transfer
Theoretically, heat transfer
efficiency from burning charcoal to cookware can be increased with costly,
complex stoves. This is rarely practical. Those who can afford such complexity
often opt for higher-status or more convenient fuels. A compromise is necessary
to achieve the best efficiency with simple, low-cost stoves usable by most
charcoal consumers.
Unlike firewood, charcoal transfers significant heat to cookware
via radiation from its glowing fuel bed. Firewood, which produces hot gases via
a slow flame, transfers heat primarily via convection.
Convective heat transfer requires hot gas to physically contact
the pot, while radiant heat transfers via infrared rays emitted directly from
the charcoal bed and absorbed by the pot’s surface. Thus, the pot must
"see" the charcoal bed to absorb radiant energy. The pot’s surface
plays a key role: it should be dull black and made of a good heat conductor.
Fire-blackened thin aluminum is optimal, while thick, low-density pottery is the worst.
How Charcoal Burns
Charcoal reacts with atmospheric oxygen at red-hot temperatures to form colorless carbon monoxide gas, which then burns with a blue flame using additional oxygen to produce carbon dioxide. The heat released from both reactions maintains the charcoal’s red glow, radiating thermal energy. The hot carbon dioxide gas leaves the combustion zone, ideally transferring most of its heat via convection through direct contact with the cookware.
As the gas cools, it releases heat and exits into the room.
Chimneys are rarely used with charcoal, as its combustion is relatively
odorless and smokeless compared to wood or raw coal. However, unburned carbon
monoxide can escape during charcoal combustion. This gas is highly toxic,
making ventilation essential in rooms where charcoal is burned.
┌────────────────────────────┐
│ BLACKENED ALUMINUM POT │
│ (Good heat conductor) │
└────────────────────────────┘
▲ ▲
│ │
Radiant Heat │ │ Convective Heat
(Infrared rays) │ │ (Hot gases)
│ │
╔══════════════════╗
║ GLOWING RED ║ ← Charcoal Briquettes
║ CHARCOAL BED ║
╚══════════════════╝
▲ ▲
│ └─── Carbon Dioxide (CO₂)
└────────── Carbon Monoxide (CO - toxic gas)
Basic Stove Body (low-cost, open or semi-vented)
Key
Points:
- Radiant heat: Directly from the glowing charcoal, absorbed best when the pot can "see" the charcoal.
- Convective heat: Comes from hot gases rising and touching the pot bottom.
- Best pot: Thin, blackened aluminum.
- Combustion: Charcoal burns with limited flame, emitting CO → CO₂ and heat.
- Ventilation: Important to avoid buildup of CO gas indoors.